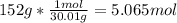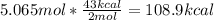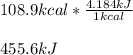Chemistry, 11.02.2021 14:00 santiagoagilg
One mole (mol) of nitrogen monoxide (NO) has a mass of 30.01 g. When
precisely 2 moles of NO(g) are produced in the following chemical reaction, 43
kcal of heat energy is "absorbed."
N2(g) + O2(g) → 2 NO(g), AH = +43 kcal
How much heat (in kJ) is exchanged when 152 g of NO(g) is produced?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3


Chemistry, 23.06.2019 07:00, ashleyrturner08
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
One mole (mol) of nitrogen monoxide (NO) has a mass of 30.01 g. When
precisely 2 moles of NO(g) are...
Questions in other subjects:

Mathematics, 26.06.2019 20:00

Mathematics, 26.06.2019 20:00

History, 26.06.2019 20:00



Computers and Technology, 26.06.2019 20:00

Chemistry, 26.06.2019 20:00


History, 26.06.2019 20:00

Mathematics, 26.06.2019 20:00