
Chemistry, 11.02.2021 14:00 moomoofower
How many moles of silver would precipitate out when 3.6 x 10^23 atoms of zinc are combined in silver nitrate according to the following unbalanced equation? (Balance equation first!)
Zn + AgNO3 → Zn(NO3)2 +Ag
A: 1.67 moles
B: 1.2 moles
C: 3.34 moles
D: 0.6 moles
Can someone please help me with this I don’t get it at all.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
How many moles of silver would precipitate out when 3.6 x 10^23 atoms of zinc are combined in silver...
Questions in other subjects:




Computers and Technology, 09.02.2021 01:20





English, 09.02.2021 01:20

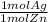 = 0.6 mol Ag
= 0.6 mol Ag


