
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δh∘=−484kj
how much pv work is done in kilojoules for the reaction of 3.20 mol of h2 with 1.60 mol of o2 at atmospheric pressure if the volume change is −22.6l?
express your answer using three significant figures

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

You know the right answer?
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δ...
Questions in other subjects:

Mathematics, 02.08.2019 10:30


English, 02.08.2019 10:30







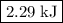 .
.
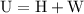
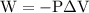
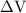 is the change in the volume in liter.
is the change in the volume in liter.
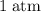 .
.
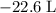 .
.
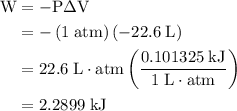
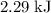 .
.


