Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
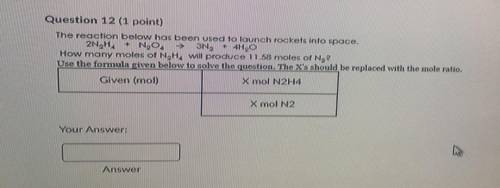
He reaction below has been used to launch rockets into space.
2N2H4 + N2O4 à 3N2 + 4H2O
Questions in other subjects:


Mathematics, 18.12.2020 18:10

Mathematics, 18.12.2020 18:10


Mathematics, 18.12.2020 18:10



Computers and Technology, 18.12.2020 18:10


Computers and Technology, 18.12.2020 18:10