
Chemistry, 10.02.2021 22:10 kiannadgarnica
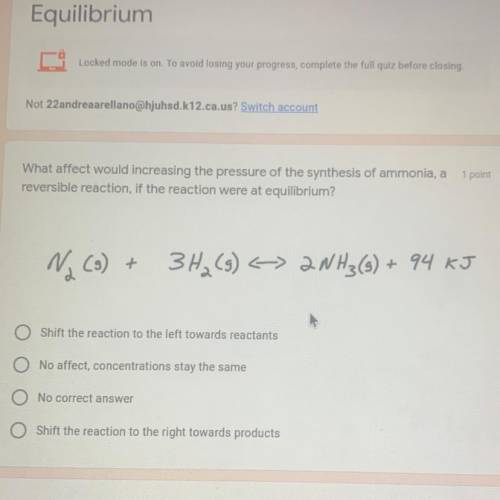
What affect would increasing the pressure of the synthesis of ammonia, a
reversible reaction, if the reaction were at equilibrium?
Na (3) +
3H₂(g) < 2NH3(s) + 94 KJ

Answers: 2


Other questions on the subject: Chemistry



You know the right answer?
What affect would increasing the pressure of the synthesis of ammonia, a
reversible reaction, if th...
Questions in other subjects:


Mathematics, 07.01.2021 18:20





Mathematics, 07.01.2021 18:20

Chemistry, 07.01.2021 18:20

Mathematics, 07.01.2021 18:20




