

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3


Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3
You know the right answer?
Using the equation below, how many liters of water can be made from 7.6 L of oxygen gas at STP?
Questions in other subjects:








Chemistry, 02.12.2020 06:00


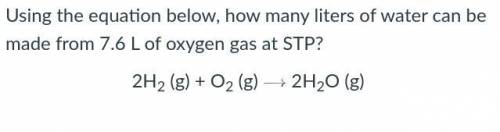
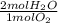 = 0.68 mol H₂O
= 0.68 mol H₂O


