Help me pleaseee
C3H8 (g) + 5 O2 (g)
->
3 CO2 (g) + 4 H2O (9)
1. Use sto...

Chemistry, 10.02.2021 20:40 natalie2sheffield
Help me pleaseee
C3H8 (g) + 5 O2 (g)
->
3 CO2 (g) + 4 H2O (9)
1. Use stoichiometry to determine how many moles of O2 are needed
to completely react with 2.25 moles of C3H8.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 00:30, boonkgang6821
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

You know the right answer?
Questions in other subjects:

History, 03.03.2021 03:00


Mathematics, 03.03.2021 03:00


Mathematics, 03.03.2021 03:00


Mathematics, 03.03.2021 03:00


Mathematics, 03.03.2021 03:00

 will be required to completely react with 2.25 moles of
will be required to completely react with 2.25 moles of 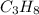 .
.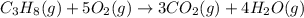
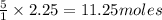 of
of 

