

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


You know the right answer?
Student was identifying the formula of 1 mole of unknown powder. When it was placed on a scale, it w...
Questions in other subjects:

Mathematics, 25.01.2020 07:31


English, 25.01.2020 07:31







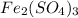
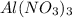 weighs = 26.98(1)+14.01(3)+15.99(9) =212.99 g/mol
weighs = 26.98(1)+14.01(3)+15.99(9) =212.99 g/mol 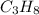 weighs = 12.01(3)+1.007(8) = 44.1 g/mol
weighs = 12.01(3)+1.007(8) = 44.1 g/mol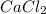 weighs = 40.07(1)+35.5(2)= 110.98 g/mol
weighs = 40.07(1)+35.5(2)= 110.98 g/mol



