

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
2C3H7OH + 9O2 -> 6CO2 + 8H2O
How Many Moles of CO2 can be produced by reacting 6 moles of C3H7OH...
Questions in other subjects:

Biology, 22.08.2019 21:30

French, 22.08.2019 21:30




Social Studies, 22.08.2019 21:30

History, 22.08.2019 21:30

History, 22.08.2019 21:30


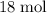
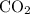 .
. 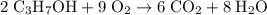 is indeed balanced.
is indeed balanced.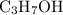 is
is 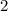 while that of
while that of 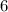 .
. 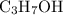 while producing
while producing 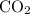 as it consumes
as it consumes 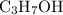 .
.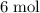 of
of 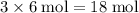 of
of 

