
Question 14
A student is titrating 83.1 g of potassium oxalate with 79.0 g of potassium permanganate. They react according to the following equation
KMnO4 + K2C2O4 → K2Mn + 2CO2 + 202
If the reaction has a 53% yield, how many grams of carbon dioxide does it produce?
А
11.79
B
44.09
23.39
2209
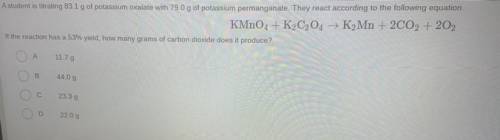

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 01:30, jusicca1109
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


You know the right answer?
Question 14
A student is titrating 83.1 g of potassium oxalate with 79.0 g of potassium permanganat...
Questions in other subjects:

Health, 20.10.2019 04:30


Biology, 20.10.2019 04:30




Social Studies, 20.10.2019 04:30


Biology, 20.10.2019 04:30




