
Chemistry, 09.02.2021 15:50 fortwill05
What is the final, balanced equation that is formed by combining these two half reactions? 2 equations: first: upper C u right arrow upper C u superscript 2 plus, plus 2 e superscript minus. Second: upper N upper O subscript 3 superscript minus, plus 2 e superscript minus, plus 2 upper H superscript plus right arrow upper n upper O subscript 2 superscript minus plus upper H subscript 2 upper O.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3


Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
What is the final, balanced equation that is formed by combining these two half reactions? 2 equatio...
Questions in other subjects:

Mathematics, 06.04.2020 01:45





Biology, 06.04.2020 01:45


English, 06.04.2020 01:46


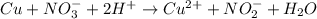
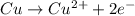 (1)
(1)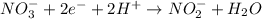 (2)
(2)

