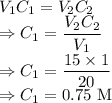Chemistry, 09.02.2021 04:20 AthenAt5607
What is the concentration in molL-1 of 20 mL of Na2CO3 solution that neutralises 15 mL of 1 M HCl solution?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, kingsqueen883
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
What is the concentration in molL-1 of 20 mL of Na2CO3 solution that neutralises 15 mL of 1 M HCl so...
Questions in other subjects:


Mathematics, 20.10.2020 20:01





Mathematics, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01


English, 20.10.2020 20:01

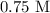
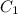 = Concentration of
= Concentration of 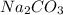
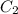 = Concentration of
= Concentration of 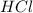 = 1 M
= 1 M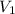 = Volume of
= Volume of 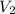 = Volume of
= Volume of