40 POINTS!
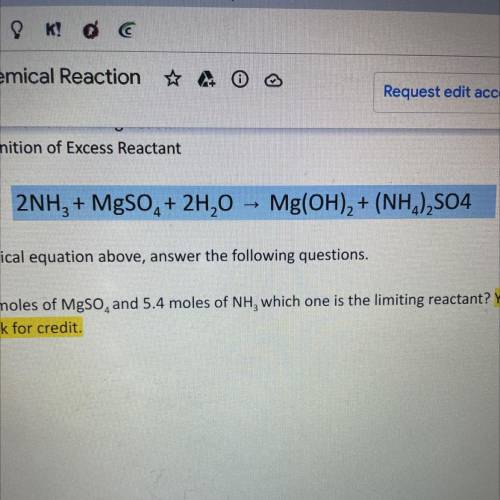
2NH3 + MgSO4 + 2H20 -> Mg(OH)2 + (NH4)2SO4
using the chemical equation ab...

Chemistry, 09.02.2021 01:30 emalvidrez5205
40 POINTS!
2NH3 + MgSO4 + 2H20 -> Mg(OH)2 + (NH4)2SO4
using the chemical equation above answer the following questions. SHOW ALL WORK OR RECEIVE NO CREDIT
A. If I have 4.6 moles of MgSO4 and 5.4 moles of NH3 which one is the limiting reactant? Show work for credit
B. What is the greatest amount of Mg(OH)2 that can be made with 4.6 moles of MgSO4 and 5.4 moles of NH3? Show work for credit
C. How many moles of the excess reactant is left over after the reaction has been completed? Show work for credit

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Questions in other subjects:



Biology, 08.02.2021 20:00


Mathematics, 08.02.2021 20:00

Mathematics, 08.02.2021 20:00


English, 08.02.2021 20:00


English, 08.02.2021 20:00



