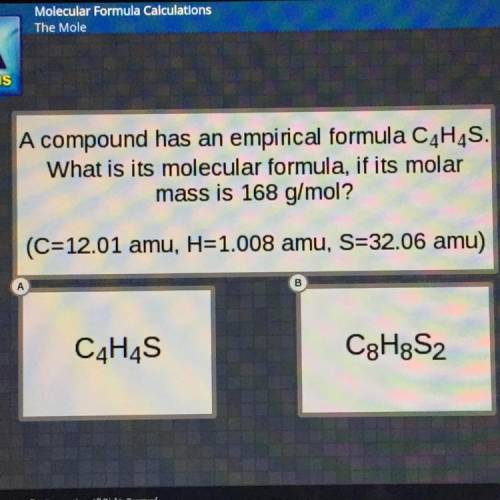
A compound has an empirical formula C4H4S.
What is its molecular formula, if its molar
mass i...

Chemistry, 09.02.2021 01:00 natalyarenassalgado
A compound has an empirical formula C4H4S.
What is its molecular formula, if its molar
mass is 168 g/mol?
(C=12.01 amu, H=1.008 amu, S=32.06 amu)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 22:30, needhelpasap8957
Why is the bottom layer of a trophic pyrimid the
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 19.05.2021 17:40

Mathematics, 19.05.2021 17:40

Mathematics, 19.05.2021 17:40

Computers and Technology, 19.05.2021 17:40



Mathematics, 19.05.2021 17:40





