
Chemistry, 08.02.2021 20:30 SophieStar15
ANSWER ASAP
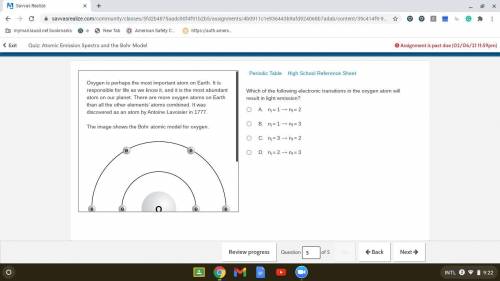
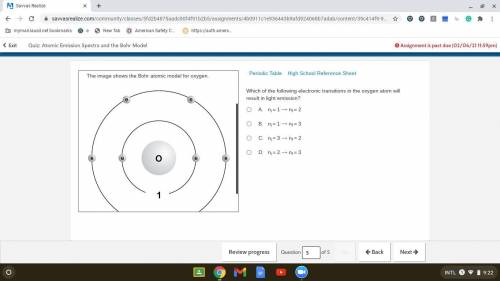
Which of the following electronic transitions in the oxygen atom will result in light emission?
. A. ni = 1 ⟶ nf = 2
B. ni = 1 ⟶ nf = 3
C. ni = 3 ⟶ nf = 2
D. ni = 2 ⟶ nf = 3

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

You know the right answer?
ANSWER ASAP
Which of the following electronic transitions in the oxygen atom will result in light e...
Questions in other subjects:


English, 04.05.2021 16:10

SAT, 04.05.2021 16:10


World Languages, 04.05.2021 16:10



Mathematics, 04.05.2021 16:10

Mathematics, 04.05.2021 16:10



