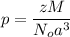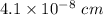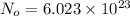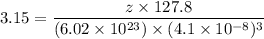Chemistry, 08.02.2021 19:20 SumayahAminaAnsari
A hypothetical AX type of ceramic material is known to have a density of 3.15 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.41 nm. The atomic weights of the A and X elements are 90.5 and 37.3 g/mol, respectively. On the basis of this information, which one of the following crystal structures is possible for this material?
a. Sodium chloride
b. Cesium chloride
c. Zinc blende

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3


You know the right answer?
A hypothetical AX type of ceramic material is known to have a density of 3.15 g/cm3 and a unit cell...
Questions in other subjects:


Mathematics, 03.12.2020 06:50

English, 03.12.2020 06:50

Mathematics, 03.12.2020 06:50


Health, 03.12.2020 06:50


Mathematics, 03.12.2020 06:50