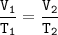Chemistry, 06.02.2021 07:50 jaimejohnston2
An arctic weather balloon is filled with 4.96 L of helium gas inside a prep shed. The temperature inside the shed is 6. °C. The balloon is then taken outside, where the temperature is 2. °C. Calculate the new volume of the balloon.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1


Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
An arctic weather balloon is filled with 4.96 L of helium gas inside a prep shed. The temperature in...
Questions in other subjects:

History, 28.04.2021 19:10




English, 28.04.2021 19:10


Geography, 28.04.2021 19:10

Mathematics, 28.04.2021 19:10

History, 28.04.2021 19:10

Chemistry, 28.04.2021 19:10