Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 09:00, boxergirl2062
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3
You know the right answer?
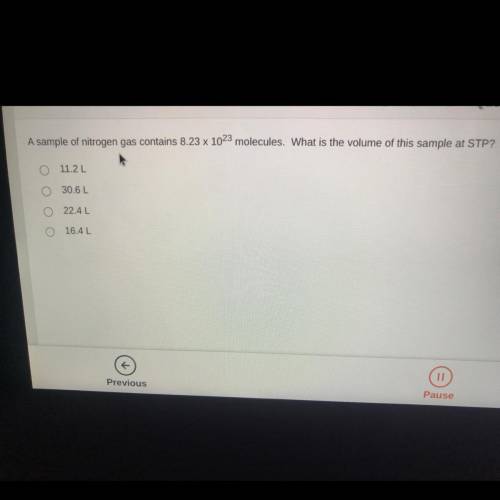
A sample of nitrogen gas contains 8.23 x 10^23 molecules. What is the volume of this sample at STP?...
Questions in other subjects:




Mathematics, 04.02.2020 02:52

Health, 04.02.2020 02:52


Mathematics, 04.02.2020 02:52



Mathematics, 04.02.2020 02:52