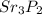Chemistry, 06.02.2021 04:20 collegebound3506
Identify the components of the ionic formula based on the name strontium phosphide. What is the symbol for the element that forms the positive cation? What is the symbol for the element that forms the negative anion? What is the subscript on the cation in the neutral formula? What is the subscript on the anion in the neutral formula?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3


You know the right answer?
Identify the components of the ionic formula based on the name strontium phosphide. What is the symb...
Questions in other subjects:

English, 11.04.2020 04:23









History, 11.04.2020 04:23

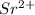 . The symbol for the element that forms the negative anion is
. The symbol for the element that forms the negative anion is 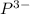 . The subscript on the cation in the neutral formula is 3 and the subscript on the anion in the neutral formula is 2.
. The subscript on the cation in the neutral formula is 3 and the subscript on the anion in the neutral formula is 2.