
Chemistry, 05.02.2021 20:10 lovelyheart5337
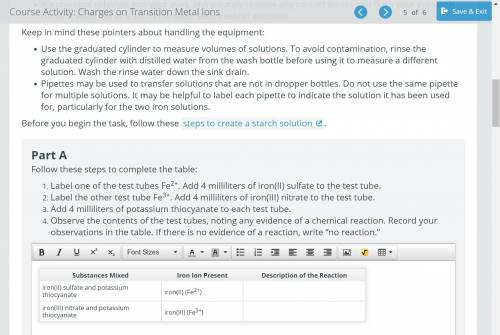
Label one of the test tubes Fe2+. Add 4 milliliters of iron(II) sulfate to the test tube.
Label the other test tube Fe3+. Add 4 milliliters of iron(III) nitrate to the test tube.
Add 4 milliliters of potassium thiocyanate to each test tube.
Observe the contents of the test tubes, noting any evidence of a chemical reaction. Record your observations in the table. If there is no evidence of a reaction, write “no reaction.”

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Label one of the test tubes Fe2+. Add 4 milliliters of iron(II) sulfate to the test tube.
Label the...
Questions in other subjects:

History, 16.09.2021 17:00


Biology, 16.09.2021 17:00



Computers and Technology, 16.09.2021 17:00

Computers and Technology, 16.09.2021 17:00

Computers and Technology, 16.09.2021 17:00

History, 16.09.2021 17:00




