Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1


Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 24.06.2019 00:00, allison9746
Ascientist performs an experiment where she measures the mass of a piece of metal, pours acid onto the metal, washes the metal, and then re-measures the mass of the metal. the scientist repeats the experiment several times, each time increasing the amount of acid used. the dependent variable in the experiment is the: initial mass of the metal final mass of the metal amount of acid used washing the metal
Answers: 1
You know the right answer?
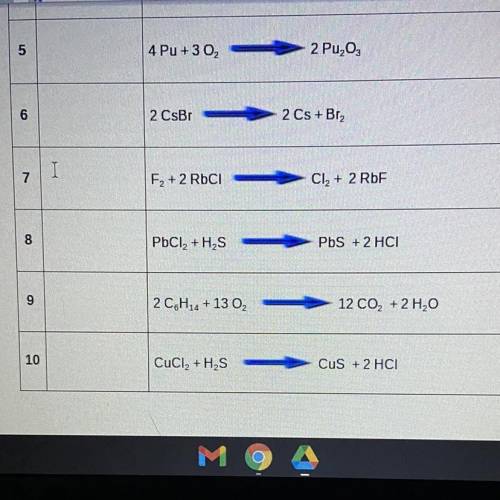
classify the following equations as : synthesis (s), decomposition (d), single replacement (sr), dou...
Questions in other subjects:

Geography, 31.08.2019 00:30


Physics, 31.08.2019 00:30






Biology, 31.08.2019 00:30

Social Studies, 31.08.2019 00:30