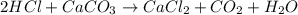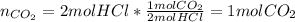Answers: 3


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, nuggetslices
Explain the effects of nh3 and hcl on the cuso4 solution in terms of le chatelier's principle
Answers: 2


Chemistry, 21.06.2019 22:30, larreanathalie3523
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 00:00, 2024daisjavien
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3
You know the right answer?
How many moles of carbon dioxide are produced in the reaction between hydrochloric acid and calcium...
Questions in other subjects:

Health, 20.07.2019 06:20

Social Studies, 20.07.2019 06:20

Health, 20.07.2019 06:20

Physics, 20.07.2019 06:20

Mathematics, 20.07.2019 06:20




Mathematics, 20.07.2019 06:20

Chemistry, 20.07.2019 06:20