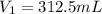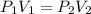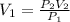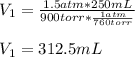Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2


Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Fluorine exerts a pressure of 900. torr. When the pressure is changed to 1.5 atm, its
volume is 250...
Questions in other subjects:

Mathematics, 08.07.2021 21:00

Mathematics, 08.07.2021 21:00








Mathematics, 08.07.2021 21:10