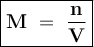Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, shahedalahmad2017
What is common about these molecules? a. their atoms are held together by covalent bonds. b. they are all made up of the same two atoms. c. their atoms are held together by ionic bonds. d. they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 21.06.2019 17:50, kelli151
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 21.06.2019 23:00, dice50
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
The molecular weight of KMnO4 is 158 g/mol. If you wanted to make 500 mL of a 0.1M stock solution of...
Questions in other subjects:

Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01






Geography, 16.10.2020 18:01