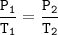Chemistry, 02.02.2021 20:20 anastasiasam1916
A certain amount of gas at 25°C and at a pressure of 0.800 atm is contained in a glass vessel. Suppose that the vessel can withstand a pressure of 2.00 atm. What is the maximum temperature in Kelvin that the gas can have without bursting the vessel?
a. 745 K
b. 62.5 K
c. 298 K
d. 760 K

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1


Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 23.06.2019 01:30, joyelewis58
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
A certain amount of gas at 25°C and at a pressure of 0.800 atm is contained in a glass vessel. Suppo...
Questions in other subjects:

Mathematics, 19.01.2021 01:50




Mathematics, 19.01.2021 02:00




English, 19.01.2021 02:00