
Chemistry, 02.02.2021 20:20 StateChamps
What is the temperature change that 725 g of aluminum will undergo when 2.35 x 104 J of thermal energy are added to it?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
What is the temperature change that 725 g of aluminum will undergo when 2.35 x 104
J of thermal ene...
Questions in other subjects:

SAT, 21.12.2020 14:00

English, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00



Chemistry, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00

Business, 21.12.2020 14:00

English, 21.12.2020 14:00

Health, 21.12.2020 14:00

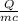 =
= 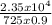 = 36°C
= 36°C

