
Chemistry, 02.02.2021 09:30 kinglightskin2k
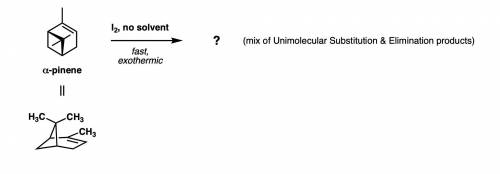
An instructor will demonstrate how quickly turpentine (paint thinner, a ~2:1 mixture of alpha and beta pinene) reacts with iodine (I2) to give a large exotherm. Draw a curvy arrow-pushing mechanism that shows the formation of one SN1 substitution product and one E1 elimination product. Keep in mind that the bottom side of the alkene, as drawn, is less hindered!

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, faithrawlins14
Which statement best describes how atoms combine to form sodium chloride (nacl)? a. a positively charged sodium ion and a positively charged chlorine ion form an covalent bond. b. a positively charged sodium ion and a negatively charged chlorine ion form an covalent bond. c. a positively charged sodium ion and a positively charged chlorine ion form an ionic bond. d. a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond.
Answers: 1

Chemistry, 21.06.2019 19:30, angelinadhar
What are the percent by mass of copper in penny lab
Answers: 3

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3
You know the right answer?
An instructor will demonstrate how quickly turpentine (paint thinner, a ~2:1 mixture of alpha and be...
Questions in other subjects:

Computers and Technology, 12.11.2019 22:31


Mathematics, 12.11.2019 22:31









