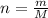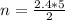Chemistry, 02.02.2021 04:00 ExperimentsDIYS
2. The heat from an acetylene torch is produced by burning acetylene (C2H2) in oxygen.
2C2H2 + 502 --> 4CO2 + 2H20
When 2.40mol C2H2 reacts with 7.40mol O2,
a. Which reactant is the limiting reactant?
b. How many grams of water can be produced by the reaction?
c. How many moles of the excess reactant remains?
I NEED THIS ANSWER QUICKLY IF YOU CAN ONLY DO B THATS FINE THANK YOU

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
2. The heat from an acetylene torch is produced by burning acetylene (C2H2) in oxygen.
2C2H2 + 502...
Questions in other subjects:

History, 30.06.2019 08:00

Mathematics, 30.06.2019 08:00

French, 30.06.2019 08:00

Mathematics, 30.06.2019 08:00


English, 30.06.2019 08:00


Mathematics, 30.06.2019 08:00


Mathematics, 30.06.2019 08:00

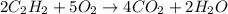
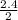 = 1.2
= 1.2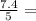 1.48
1.48