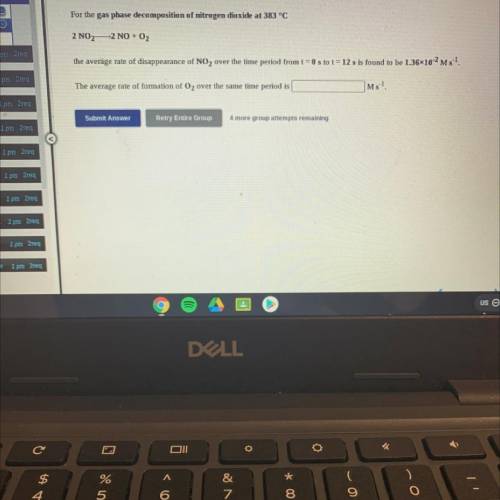
For the gas phase decomposition of nitrogen dioxide at 383 °C
2 NO2—2 NO + O2
the average rat...

Chemistry, 02.02.2021 03:20 tyneshiajones124
For the gas phase decomposition of nitrogen dioxide at 383 °C
2 NO2—2 NO + O2
the average rate of disappearance of NO2 over the time period from t = 0 s to t = 12 s is found to be 1.3610-2 Ms 1.
The average rate of formation of O2 over the same time period is
Ms-1

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2

Chemistry, 23.06.2019 07:20, prettydoll19
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3

Chemistry, 23.06.2019 09:00, alisonlebron15
Are the results of a thoroughly tested hypothesis?
Answers: 2
You know the right answer?
Questions in other subjects:

Social Studies, 06.11.2020 07:30


English, 06.11.2020 07:30

History, 06.11.2020 07:30

English, 06.11.2020 07:30


Mathematics, 06.11.2020 07:30

History, 06.11.2020 07:30

Chemistry, 06.11.2020 07:30

Physics, 06.11.2020 07:30



