
Chemistry, 02.02.2021 03:10 janellball16
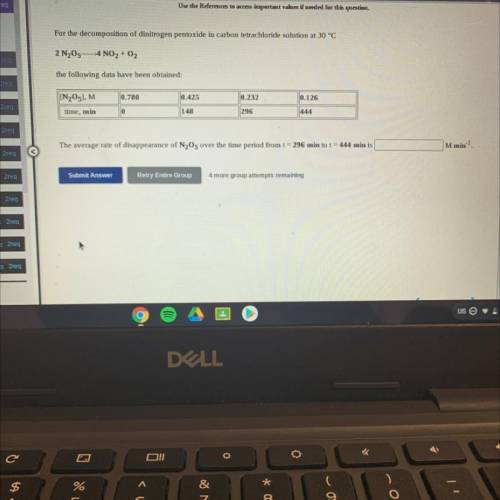
For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C
2 N205-4 NO2 + O2
the following data have been obtained:
[N205], M
0.780
0.425
0.232
0.126
||444
time, min
0
148
296
M min-1
The average rate of disappearance of N2O5 over the time period from t = 296 min to t = 444 min is

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 23.06.2019 03:00, rhianna18
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1

Chemistry, 23.06.2019 07:00, SMURFETTE86
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
You know the right answer?
For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C
2 N205-4 NO...
Questions in other subjects:


Business, 16.10.2020 03:01



History, 16.10.2020 03:01


English, 16.10.2020 03:01






