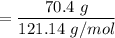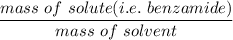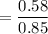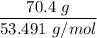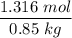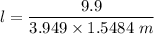Chemistry, 01.02.2021 20:40 alyonaprotopopova
When 70.4 g of benzamide (C7H7NO) are dissolved in 850. g of a certain mystery liquid X, the freezing point of the solution is 2.7 C lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (NH CI) are dissolved in the same mass of X, the freezing point of the solution is 9.9 °C lower than the freezing point of pure X.
Required:
Calculate the van't Hoff factor for ammonium chloride in X.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, 20heldmadison
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1

Chemistry, 21.06.2019 18:30, mamasmontoya
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
When 70.4 g of benzamide (C7H7NO) are dissolved in 850. g of a certain mystery liquid X, the freezin...
Questions in other subjects:

History, 16.12.2020 23:00

History, 16.12.2020 23:00

Business, 16.12.2020 23:00




Mathematics, 16.12.2020 23:00

Advanced Placement (AP), 16.12.2020 23:00

Mathematics, 16.12.2020 23:00

Mathematics, 16.12.2020 23:00