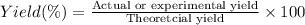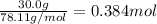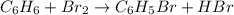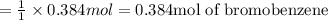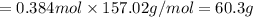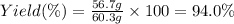Chemistry, 31.01.2021 04:40 ligittiger12806
1.What is the theoretical yield of C6H5Br in this reaction when 30.0 g of C6H6 reacts with excess Br2? If the actual yield of C6H5Br was 56.7 g, what is the percent yield?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
1.What is the theoretical
yield of C6H5Br in this reaction when 30.0 g of C6H6
reacts with excess B...
Questions in other subjects:

Mathematics, 02.09.2021 14:00




English, 02.09.2021 14:00

Mathematics, 02.09.2021 14:00


Mathematics, 02.09.2021 14:00