
Chemistry, 30.01.2021 16:10 kaylee0424
PLEASE HELP ME
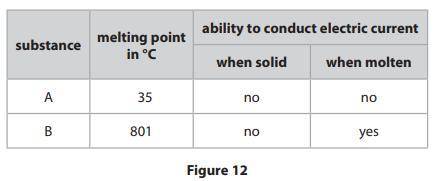
Figure 12 shows the melting points of two substances, A and B, and the abilities
of the substances to conduct an electric current when solid and when molten.
One of the substances has an ionic structure and one has a simple molecular,
covalent structure.
Explain, in terms of bonding and the forces between the particles, the relative
melting points and abilities to conduct the electric current of substances A and B.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1



Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
PLEASE HELP ME
Figure 12 shows the melting points of two substances, A and B, and the abilities
Questions in other subjects:

Chemistry, 01.10.2019 14:00







Health, 01.10.2019 14:00


History, 01.10.2019 14:00



