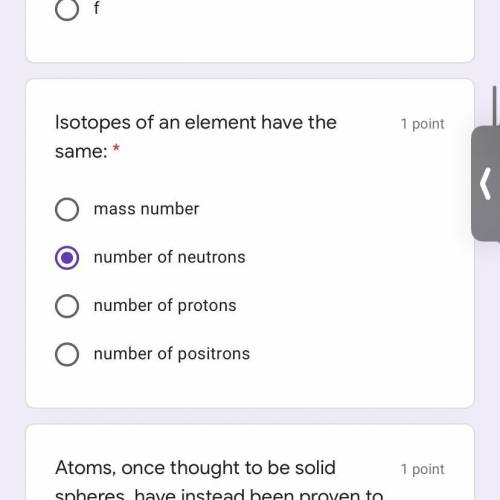
I need help with this
...

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, jrjimenez
At 40 âc the solution has at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water and it can contain up to at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water. at 0 âc the solubility is ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. kno3 per 100 g of water, so ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. gkno3 per 100 g of water will precipitate out of solution. a kno3 solution containing 55 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 2


Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Questions in other subjects:


Physics, 04.09.2020 07:01

History, 04.09.2020 07:01

Physics, 04.09.2020 07:01

Mathematics, 04.09.2020 07:01


Mathematics, 04.09.2020 07:01

Mathematics, 04.09.2020 07:01

Mathematics, 04.09.2020 07:01

English, 04.09.2020 07:01