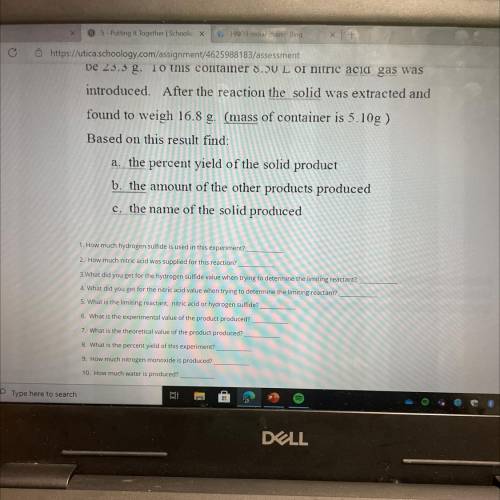
Elemental sulfur is one of the products of the gas-phase
reaction of nitric acid and hydrogen...

Chemistry, 30.01.2021 07:30 jnsebastian2002
Elemental sulfur is one of the products of the gas-phase
reaction of nitric acid and hydrogen sulfide. The other
products are nitrogen monoxide(g) and water(g). A
container and hydrogen sulfide (s) are massed and found to
be 23.3 g. To this container 8.50 L of nitric acid gas was
introduced. After the reaction the solid was extracted and
found to weigh 16.8 g. (mass of container is 5.10g )
Based on this result find:
a. the percent yield of the solid product
b. the amount of the other products produced
c. the name of the solid produced
1 How much hydrogen sulfide is used in this experiment?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, mgnbrnne
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Questions in other subjects:



Biology, 20.08.2019 01:40



Mathematics, 20.08.2019 01:40



Mathematics, 20.08.2019 01:40

Social Studies, 20.08.2019 01:40



