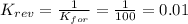I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure would
N2(g)+3H2(g)⇄2NH3(g)
a. increase [H2]
b. increase [NH3]
c. increase [N2]
d. have no effect
2. If K=100, then the value of K for the reverse reaction is
a. the same value
b. can only be determined by experimentation
c. the negative of the value for the forward reaction
d. 0.01

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure woul...
Questions in other subjects:

Computers and Technology, 27.01.2020 21:31