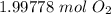CH4 + 202 → CO2 + 2H2O
How many moles of O2, needed to produce 36 grams of H20?...

Chemistry, 30.01.2021 06:00 kittycat92
CH4 + 202 → CO2 + 2H2O
How many moles of O2, needed to produce 36 grams of H20?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3


Chemistry, 23.06.2019 03:30, rniadsharri16
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 02.03.2020 09:15





Mathematics, 02.03.2020 09:16


Computers and Technology, 02.03.2020 09:16


Computers and Technology, 02.03.2020 09:16

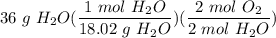 Divide/Multiply:
Divide/Multiply: