Chemistry, 30.01.2021 02:30 lydiakegg454
A sample of blood plasma occupies 0.550 L at 0'C and 1.03 bar, and is compressed isothermally by 0.57 per cent by being subjected to a constant external pressure 95.2 bar. Calculate w.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, viktoria1198zz
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1


Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
A sample of blood plasma occupies 0.550 L at 0'C and 1.03 bar, and is compressed isothermally by 0.5...
Questions in other subjects:





Mathematics, 19.07.2019 15:00


History, 19.07.2019 15:00

Health, 19.07.2019 15:00


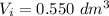 at a temperature of 0° C. In the isothermal expansion of its sample data in a
at a temperature of 0° C. In the isothermal expansion of its sample data in a 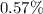 reduction of its size; the actual sample size is thus
reduction of its size; the actual sample size is thus![V_f = [(0.550) - 0.57 \% \ of (0.550)] dm^3](/tpl/images/1079/4783/510d1.png)
![= [(0.550)-(\frac{0.57}{100}) \times (0.550)] \ dm^3 \\\\= [(0.550)-(0.0057) \times (0.550)] \ dm^3 \\\\= [0.550-0.003135] \ dm^3 \\\\= 0.546865 \ dm^3](/tpl/images/1079/4783/59aa1.png)
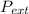 was =
was =  . It is the job completed is, therefore,
. It is the job completed is, therefore,