PLEASE ANSWER QUICKLY. TYSM
Using the diagram above, answer the following questions:
Tr...

PLEASE ANSWER QUICKLY. TYSM
Using the diagram above, answer the following questions:
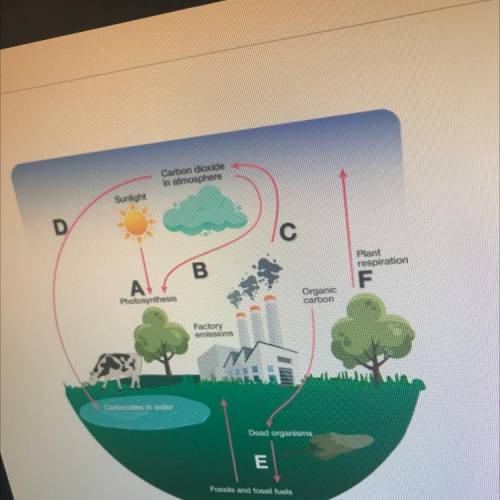
True or False. The arrow labeled C represents a transfer of chemical energy to
mechanical energy. Explain why this is true or false.
•••••••••••••••••••••••••••••••••
True or False. The arrow labeled A represents a transfer of solar energy to chemical energy. Explain why this is true or false.
•••••••••••••••••••••••••••••••Whic h arrow or arrows represent a release of carbon dioxide? What process is occurring
at the arrow(s) you selected?
•••••••••••••••••••••••••••••••••Wh ich arrow or arrows indicate a process that cycles carbon from living or nonliving
organisms? Describe the process or processes you selected.
•••••••••••••••••••••••••••••••••
Which arrow or arrows represent reactions that demonstrate a conservation of mass and
energy? Explain your answer.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:50, strawberrymrmr756
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 15.10.2020 04:01

History, 15.10.2020 04:01

English, 15.10.2020 04:01



Biology, 15.10.2020 04:01


Mathematics, 15.10.2020 04:01



