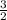Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1


Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
Iron metal reacts with elemental oxygen to form rust according to the unbalanced reaction, Fe(s) + O...
Questions in other subjects:


Social Studies, 18.07.2019 11:40


Business, 18.07.2019 11:40

History, 18.07.2019 11:40




Chemistry, 18.07.2019 11:40