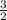Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:50, BookandScienceNerd
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
Iron metal reacts with elemental oxygen to form rust according to the unbalanced reaction, Fe(s) + O...
Questions in other subjects:

English, 14.08.2020 05:01

Mathematics, 14.08.2020 05:01



History, 14.08.2020 05:01