2) The decomposition of an unknown molecule, A, was found to be a first order
process.
a. The...

Chemistry, 29.01.2021 18:00 lordcaos066
2) The decomposition of an unknown molecule, A, was found to be a first order
process.
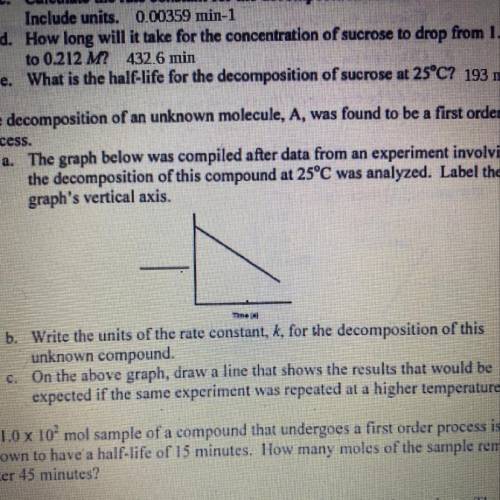
a. The graph below was compiled after data from an experiment involving
the decomposition of this compound at 25°C was analyzed. Label the
graph's vertical axis.
Time
b. Write the units of the rate constant, k, for the decomposition of this
unknown compound.
c. On the above graph, draw a line that shows the results that would be
expected if the same experiment was repeated at a higher temperature.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
Questions in other subjects:



Biology, 10.02.2021 21:10

Mathematics, 10.02.2021 21:10



Mathematics, 10.02.2021 21:10



Mathematics, 10.02.2021 21:10



