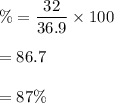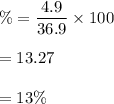Chemistry, 29.01.2021 16:30 cynayapartlow88
Large amounts of an unknown compound were isolated from seawater. The compound contained 32.0 grams ofbromine and 4.9 grams of magnesium. What is its percent composition?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, ayahabdulhaqq2
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3


Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
Large amounts of an unknown compound were isolated from seawater. The compound contained 32.0 grams...
Questions in other subjects:

Advanced Placement (AP), 27.04.2020 03:05

Mathematics, 27.04.2020 03:05

Mathematics, 27.04.2020 03:05

Mathematics, 27.04.2020 03:05

Mathematics, 27.04.2020 03:05

Mathematics, 27.04.2020 03:05


History, 27.04.2020 03:05

Mathematics, 27.04.2020 03:05