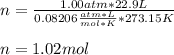Chemistry, 29.01.2021 14:00 aisatubrodie4626
50POINTS!
Using the ideal gas law (PV=nRT) solve for the missing. Variable. R= 0.08206atm*L/mol*k
If 22.9L of an ideal gas was collected at STP. How many moles of the gas were present?
A. 1.02 moles
B. 5.99 moles
C. 3.05 moles
D. 2.74 moles


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3


Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
50POINTS!
Using the ideal gas law (PV=nRT) solve for the missing. Variable. R= 0.08206atm*L/mol*k
Questions in other subjects:

English, 28.01.2021 17:50



Mathematics, 28.01.2021 17:50


History, 28.01.2021 17:50

Chemistry, 28.01.2021 17:50


Mathematics, 28.01.2021 17:50