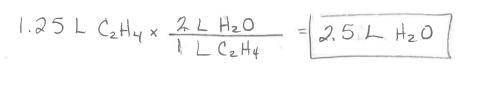Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
Ethylene burns in oxygen to form carbon dioxide and water vapor:
C2H4(g) + 3 02(g) --> 2 CO2(g)...
Questions in other subjects:

Mathematics, 01.03.2021 18:10




Mathematics, 01.03.2021 18:10

Mathematics, 01.03.2021 18:10

Advanced Placement (AP), 01.03.2021 18:10


Mathematics, 01.03.2021 18:10