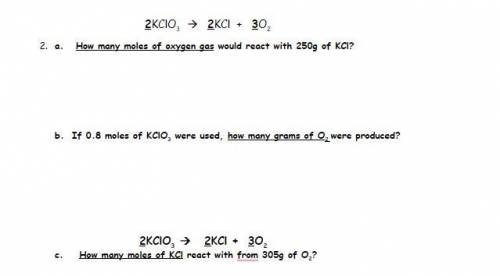
2KClO3 2KCl + 3O2
2 - A. How many moles of oxygen gas would react with 250g of KCl?
2 -...

Chemistry, 29.01.2021 03:30 shortty1111
2KClO3 2KCl + 3O2
2 - A. How many moles of oxygen gas would react with 250g of KCl?
2 - B. If 0.8 moles of KClO3 were used, how many grams of O2 were produced?
2- C. How many moles of KCl react with 305g of O2?
There are multiple questions for the chemical equation, so I've just combined them all into one.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mauifrifer3986
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:30, Clivensp5
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils. the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
Questions in other subjects:





Advanced Placement (AP), 15.04.2020 11:46


Mathematics, 15.04.2020 11:47

Mathematics, 15.04.2020 11:47

Law, 15.04.2020 11:47




