Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 02:30, hailee232
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
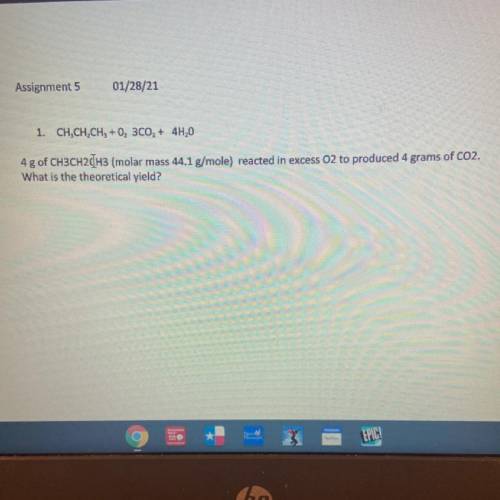
1. CH, CH, CH3 + 0, 300, + 4H,0
4g of CH3CH2CH3 (molar mass 44.1 g/mole) reacted in excess O2 to pr...
Questions in other subjects:


Mathematics, 20.11.2020 02:30


Computers and Technology, 20.11.2020 02:30

History, 20.11.2020 02:40


History, 20.11.2020 02:40

Mathematics, 20.11.2020 02:40

Mathematics, 20.11.2020 02:40