Consider the equations below.
CH4 (g)->C(s)+2H2(g) H1 = 74.6 kJ
C(s) + 2CI2(g)->CC...

Chemistry, 28.01.2021 06:40 zoelynn8386
Consider the equations below.
CH4 (g)->C(s)+2H2(g) H1 = 74.6 kJ
C(s) + 2CI2(g)->CCI4(g) H2 = -95.7 kJ
2H2(g) + 2CI2(g)-> 4HCI(g) H3 = -184.6 kJ
CH4(g) + 4CI2(g) -> CCI4(g) + 4HCI(g) H4 = -205.7 kJ
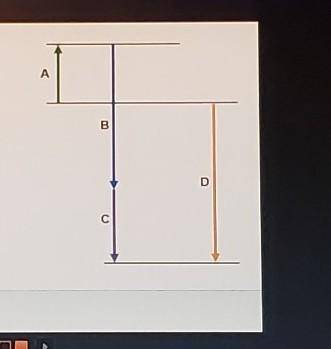
Complete the following based on the diagram.
Arrow A: 74.6 kJ
-95.7 kJ
-184.6 kJ
Arrow B: endothermic
exothermic
Arrow C: - bas a magnitude that is greater than that of B
- has a magnitude that is less than that of B
- has negative enthalpy
Arrow D: - represents an intermediate reaction
- has a magnitude that is always higher than any intermediate reaction
- represents the overall enthalpy of reaction

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Questions in other subjects:


English, 10.10.2019 04:30

History, 10.10.2019 04:30


Mathematics, 10.10.2019 04:30



History, 10.10.2019 04:30

Mathematics, 10.10.2019 04:30





