
Chemistry, 27.01.2021 20:40 GachaSkylarUwU
A main group element with the valence electron configuration 3s23p1 is in periodic group . It forms a monatomic ion with a charge of . A main group element with the valence electron configuration 4s24p5 is in periodic group . It forms a monatomic ion with a charge of .

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 19:20, johnkings140
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
A main group element with the valence electron configuration 3s23p1 is in periodic group . It forms...
Questions in other subjects:











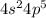

 main group component to valence electronic structure (IIIA group) and Aluminum (Al). The monatomic ion forms a load of (+3)
main group component to valence electronic structure (IIIA group) and Aluminum (Al). The monatomic ion forms a load of (+3) (Cl). It constitutes a monatomic ion with a load of (-1).
(Cl). It constitutes a monatomic ion with a load of (-1).

