
(05.06) % Yield Lab Report
1. Write the balanced chemical equation for the reaction you’re performing
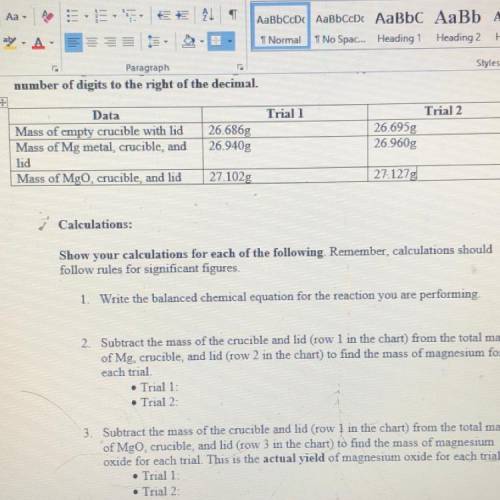
2. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of Mg, crucible, and kid (row 2 in the chart) to find the mass of magnesium for each trial
Trial 1:
Trial 2:
3. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of MgO, crucible, and lid (row 3 in the chart) to find the mass of magnesium oxide for each trial. This is the actual YIELD of magnesium oxide for each trial
Trial 1:
Trial 2:
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
Trial 1:
Trial 2:
5. Determine the percent yield of MgO for your experiment for each trial
Trial 1:
Trial 2:
6. Determine the average percent yield of MgO for 2 Trials
CONCLUSION
Write a conclusion statement that addresses the following questions
• Explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
• What sources of error may have contributed to the percent yield not being 100%?
(Think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.)
• How do you think the investigation can be explored further?
POST LAB REFLECTION QUESTIONS
1. When conducting this experiment, some procedures call for heating the substance several times and recording the mass after each heating, continuing until the mass values are constant. Explain the purpose of this process and how it might reduce errors.
2. Your company currently uses a process with a similar cost of materials that has an average percent yield of 91%. If the average percent yield of this process is higher than that, this could save the company money. What is your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, 20jessicacabriales
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1


Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1
You know the right answer?
(05.06) % Yield Lab Report
1. Write the balanced chemical equation for the reaction you’re performi...
Questions in other subjects:



Biology, 21.03.2021 04:20

Mathematics, 21.03.2021 04:20



Mathematics, 21.03.2021 04:20





